Understanding Past Climate: Ice Core Analysis
Ice core analysis is a fascinating scientific method that allows us to travel back in time and understand the past climatic conditions of our planet. By studying ice cores, scientists can determine past temperatures and concentrations of carbon dioxide (CO2) in the atmosphere. But how is this possible? This article takes you on a scientific journey to understand the process.
What is an ice core?
An ice core is a sample of ice that is taken by drilling into ice caps and glaciers. These ice cores contain tiny air bubbles that are trapped when snow turns into ice. These air bubbles are like little time capsules, preserving the air from different eras of Earth's history.
How are ice cores analyzed?
Ice cores are analyzed by measuring the isotopic composition of the water and the concentration of greenhouse gases in the air bubbles. The oxygen isotopes in the water molecule give us information about the temperature when the snow fell. The air bubbles contain greenhouse gases, such as carbon dioxide (CO2), which can be measured to determine past concentrations of these gases.
The oxygen isotopes in the water molecule are a valuable tool for understanding past temperature variations. Here's how it works: Oxygen has three natural isotopes: ^16O, ^17O, and ^18O. The most abundant is ^16O, but ^18O is also quite common. When water evaporates from the ocean, molecules containing the lighter isotope (^16O) tend to evaporate a bit more easily than those containing the heavier isotope (^18O). This means that the water vapor in the atmosphere has a higher proportion of ^16O relative to ^18O than ocean water. When this water vapor condenses and falls as snow on the ice caps, it preserves this proportion of isotopes. However, the exact proportion of ^16O to ^18O in the snow depends on the temperature. When it's warmer, a greater proportion of ^18O evaporates from the ocean and ends up in the snow. When it's colder, a greater proportion of ^16O evaporates and ends up in the snow. Thus, by measuring the proportion of these two isotopes in the ice from an ice core, scientists can deduce the temperature at the time that snow fell. That's why the oxygen isotopes in the water molecule are such a valuable tool for studying past temperature variations.
Correlation between temperature and CO2
By analyzing ice cores, scientists have discovered a close correlation between past temperatures and CO2 concentrations. When CO2 concentrations are high, temperatures are generally higher. This correlation is due to the greenhouse effect, where CO2 in the atmosphere traps the sun's heat, leading to an increase in temperature.
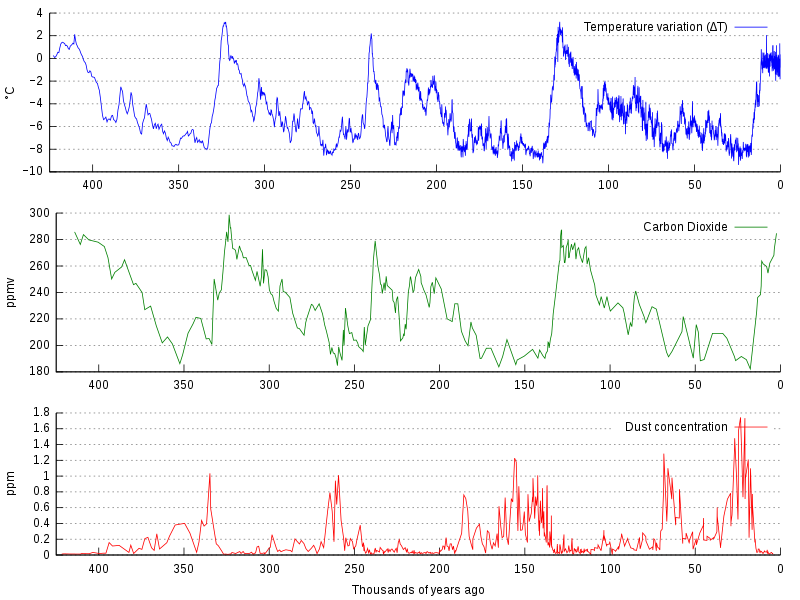 Graph of CO2 (green), reconstructed temperature (blue) and dust (red) from the Vostok ice core
over
the last
420,000 years
Graph of CO2 (green), reconstructed temperature (blue) and dust (red) from the Vostok ice core
over
the last
420,000 years
Where are these studies conducted?
Ice core studies are conducted in places around the world where there are large expanses of ice, such as Antarctica and Greenland. Some of the main institutes conducting this research include:
- The Climate and Environment Research Institute of the University of Copenhagen, Denmark.
- The Climate Research Institute of the University of Maine, United States.
- The Alfred Wegener Institute for Polar and Marine Research, Germany.
Here are some of the main research projects studying ice cores in Antarctica:
- Byrd Station Project in Antarctica: This project reached depths of over 2164 m in the 1960s.
- Soviet Ice Drilling Projects at Vostok Station: These projects lasted several decades, with the deepest core reaching 3769 m.
- West Antarctic Ice Sheet project: This project also conducted many deep drillings in Antarctica.More information here
- Projects managed by the British Antarctic Survey and the International Trans-Antarctic Scientific Expedition: These organizations have also conducted many drillings in Antarctica. More information here
- Collaborative projects in Greenland: Although not in Antarctica, it is worth mentioning that several projects have been conducted in Greenland since the 1970s, with the most recent project, the East Greenland Ice-Core Project, originally set to complete a deep core in East Greenland in 2020.
These research projects are typically international and require years of planning and execution due to the logistical complexity of accessing these remote regions.
Conclusion
Ice core analysis is a valuable method for understanding the past climate of our planet. By studying these precious ice archives, scientists can better understand how the climate has changed over time and how it might evolve in the future.
